Abstract
INTRODUCTION: Both solid and hematologic malignancies are associated with an increased risk of thromboembolic events, according to previous studies. One of the most common risk scoring systems was developed based on type of non-hematologic cancer, body mass index (BMI), WBC, hemoglobin, and platelet counts. However, compared to other solid tumors, acute leukemia patients commonly present with pancytopenia at the time of diagnosis and the Khorana Risk Score (KRS) has not been validated in patients with acute myeloid leukemia (AML). The goal of our study was to validate the KRS in AML patients.
METHODS: Using Total Cancer Care (TCC), we retrospectively identified histologically confirmed AML patients from 2000 to 2018. Clinical and laboratory variables including age, gender, previous cancer history, use of growth factors, underlying coagulopathy, white blood cell counts, hemoglobin, and platelet counts were characterized at the time of AML diagnosis and annotated using descriptive statistics. The thrombotic event rate was estimated with the Kaplan-Meier method and compared using log-rank test. All statistical analyses were performed using SPSS v24.0 and GraphPad Prism 7.
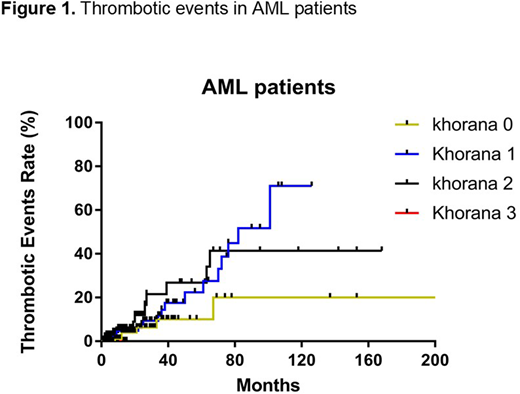
RESULTS: A total of 867 AML patients were included in this study. The median age at AML diagnosis was 75 (51-96) years and male patients were 65% (n=565). A total of 28% (n=241) patients had prior cancer history (hematologic malignancies, n=34; solid tumor, n=207). A total of 12% (n=101), 7% (n=58), and 1% (n=8) patients had EPO, G-CSF, GM-CSF treatment prior to AML diagnosis and 84% (n=728) patient were treated with any typed of growth factors after AML diagnosis. The median BMI was 27.1 (14.8-103) and 5% (n=47) patients had BMI higher than 35. A total of 26% (n=229) patients had WBC ≥11 x109/L (median 3.165 (0.08-413.74) x109/L), 67% (n=584) patients had hemoglobin <10 g/L (median 9.3 (5.6-15.2) g/L, 1% (n=10) patients had platelet >350 x109/L (median 46 (1-800) x109/L). A total of 22% (n=191) patients had Khorana score 0, 51% (n=445) patients had score 1, 24% (n=207) patients had score 2, and 3% (n=24) patients had score 3, respectively. A total of 42 thrombotic events were observed in the median follow up of 3 (0.1-307) months. Among these, a total of 3% (n=6/191), 5% (n=23/445), 6.3% (n=13/207), and 0% (n=0/24) thrombotic events occurred in patients with Khorana sore 0, 1, 2, and 3, respectively. Log-rank (Mantel-Cox) test showed no statistical difference between individual subgroup (p=0.1949).
CONCLUSIONS: In AML patients, there was a higher incidence of thrombotic events in patients with higher Khorana score though the difference was not statistically significant. The proportion of patients with Khorana score ≥3 was relatively low, which could be due to pancytopenia, a common presentation in AML patients. These results suggest that the development of better thrombotic risk scoring system is warranted in patients with AML.
Komrokji:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.